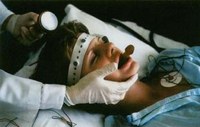
Thousands spoke out to the US Food and Drug Administration (FDA) to oppose their controversial proposal to approve devices used for electroshock — also known as electroconvulsive therapy or ECT — without any testing for safety and effectiveness. The deadline of 8 January 2010 has passed, but you can still speak out. [Update: 27 Jan. 2010]
The FDA says it may de-regulate electroshock… when it’s never regulated the device in the first place.
Update:
MindFreedom has been told that an informal count shows that more than 3,500 people commented to the FDA about their proposed reclassification of electroshock. The ratio of opposition to this reclassification was 3 to 1. THANK YOU to everyone who spoke out!
MFI NEWS RELEASE
MindFreedom Helped Reach Thousands to Oppose FDA Reclassification of ECT
January 8, 2010 was the cut off for your comments to FDA on ECT reclassification. But you can still speak out!
MindFreedom International helped reach thousands of people about speaking out to the USA Food & Drug Administration about their proposal to reclassify the device used for electroshock, also known as electroconvulsive therapy.
While the official comment period is over, you can always speak out to the FDA to let them know your point of you.
You may postal mail to:
FDA Commissioner Margaret Hamburg, M.D.
Division of Dockets Management (HFA-305)
Food and Drug Administration
5630 Fishers Lane, rm. 1061
Rockville, MD 20852
You are encouraged to say:
“I oppose the FDA’s proposed reclassification of the ECT device to Class II. The FDA should investigate the ECT device for safety. The FDA should call for Pre-Market Approval Applications for the device.”
Of course, if you can also add specific points about why electroshock devices ought to be investigated for safety and effectiveness please do so.
Background, from original alert:
MindFreedom International, an independent nonprofit coalition for human rights in the mental health system, launched a campaign to encourage widespread public input to stop the US Food & Drug Administration (FDA) from giving a rubber stamp to the device used for electroconvulsive therapy (ECT or electroshock).
Said David Oaks, Director of MindFreedom, “Electroshock devices are way less regulated than, say, the US banking system. Electroshock manufacturers are like the Bernie Madoff of mental health, operating unethically for decades without hardly any oversight. Now the FDA wants to rubber stamp the device and de-regulate electroshock even more. Everyone who is concerned should speak out, now!”
In Brief: USA Electroshock Regulation
Since the 1970’s, when federal law granted the FDA the authority to regulate medical devices in the United States, the machines used in electroconvulsive therapy (ECT) have been classified as high-risk, or ‘Class III’ devices. To protect the American public such devices are required by law to undergo a rigorous Pre-Market Approval (PMA) process in order to establish their safety and effectiveness.
This never happened with ECT devices. Instead, these machines were grandfathered in — having been in use for decades — and have never undergone the testing required of other Class III devices.
In April 2009, the FDA issued a notice indicating that it would finally review the safety and effectiveness of ECT devices for the first time ever.
Jim Gottstein of PsychRights explains the industry’s response: “The deadline for submissions has passed, but the manufacturers have not conducted any clinical trials, claiming they cannot afford them. They simply point to the opinions of shock doctors (including those who have financial interests in companies making electroshock machines) as evidence that shock is safe.”
Under pressure from the industry, the FDA appears likely to respond by downgrading ECT machines to Class II or “low risk.” If that happens, these dangerous machines might never be evaluated.
There is still time to take action!
Maybe because of outrage expressed by mental health advocates, including MindFreedom, the FDA opened up a “docket” for public comment until January 8, 2010. This could be the last official opportunity for years for concerned citizens to let the FDA know just how important it is to hold the ECT industry accountable for its claims that these devices are safe.
Linda Andre, author of the book “Doctors of Deception: What They Don’t Want You to Know About Shock Treatment” said: “For 30 years, FDA has been on record stating that ECT is a risky procedure which can cause brain damage and permanent amnesia, and the agency could have taken action at any time to protect patients, but it did not. A generation of patients has been subjected to an untested, unsafe procedure. It is far past time for the FDA to require that ECT be investigated for safety, and that means clinical trials, not selective literature reviews.”
You may purchase Linda Andre’s new book critical of electroshock, Doctors of Deception, via MindFreedom’s MAD MARKET, click here:
To take action about stopping the FDA from reclassifying the electroshock device, click here.
For more background info about electroshock, and links to more info click here.
Text of MFI Comments to FDA
Below is text from the letter that MindFreedom International submitted to the FDA:
8 January 2010
FDA Commissioner Margaret Hamburg, M.D.
Division of Dockets Management (HFA-305)
Food and Drug Administration
5630 Fishers Lane, rm. 1061
Rockville, MD 20852
Re: Docket No. FDA-2009-N-0392
Oppose Reclassification of Electroconvulsive Therapy Device
Dear Commissioner Hamburg:
On behalf of MindFreedom International, this letter is to absolutely oppose the FDA’s proposed reclassification of the device used for electroconvulsive therapy (ECT, also commonly known as electroshock) from Class III to a “low risk” designation.
The FDA should instead investigate the ECT device for safety on an urgent basis. The FDA should call for Pre-Market Approval Applications from all manufacturers.
Below please find ten specific points with citations about why the FDA should finally, after all these many decades, require an investigation of this device. We have reviewed many of the public comments already submitted to the FDA about reclassification.
MindFreedom International has, for 24 years, been one of the few totally independent coalitions in the mental health advocacy field, with no funding from the mental health industry. MindFreedom International is accredited by the United Nations as a Non-Governmental Organization (NGO) with Consultative Roster Status.
1)PROVEN HISTORY OF SERIOUS CONSUMER COMPLAINTS
The FDA’s own files over these many years reveal a history of an enormous number of heartbreaking, serious complaints – memory problems, physical suffering, psychological trauma, cognitive damage – from those who have been harmed by the ECT device.
Many of those commenting to the FDA in defense of ECT reclassification point to individuals who state they have had successful outcomes with ECT. However, this does not in any way provide evidence that the ECT device has been proven to be “low risk.”
The medical literature, of course, has many reports about severe trauma, memory problems, and cognitive difficulties caused by the ECT device.
For example, in her scholarly article, “Adverse psychological effects of ECT” published in the Journal of Mental Health (1999) 8, 1, 69±85, author Lucy Johnstone’s peer-reviewed research clearly established this existence of ECT device induced distress.
From Ms. Johnstone’s Abstract:
“Although it is known that a proportion of people find ECT distressing to receive, these adverse psychological reactions are little understood. Twenty people who reported having found ECT upsetting were interviewed about their experiences in detail. A variety of themes emerged, including feelings of fear, shame and humiliation, worthlessness and helplessness, and a sense of having been abused and assaulted. This had reinforced existing problems and led to distrust of psychiatric staff. Few had felt able to tell professionals of the strength of their reactions, implying a possible hidden pool of trauma.”
MindFreedom International has received hundreds of similar complaints from individuals who have experienced heartbreaking impacts from ECT. Because memories cannot be put under a microscope, some in the medical field have discounted such reports. In addition, because the impact of shock can be so diverse, sometimes those with positive experiences are heard more than those who have been harmed.
However, it is undeniable that many individuals have experienced severe high risk from the ECT procedure. We have heard reports of loss of marriage ceremonies, birth of children, career skills, languages, musical abilities, friends, locations, and more. We have heard of diminishment of cognitive abilities, making other psychosocial alternatives more difficult.
If the ECT device was proposed for the first time now, in 2010, clearly it would be required to undergo investigation for safety and high risk. Therefore, in that case, the long history of serious complaints by ECT recipients makes an investigation of that device that much more necessary.
It is a sad commentary on the status of people diagnosed with psychiatric disorders that, say, a new rubber glove would be more regulated by the FDA than a device which has for decades caused such suffering, memory problems, and cognitive impairment.
2)ECT DEVICE REMAINS ONE OF MOST CONTROVERSIAL IN PSYCHIATRY
Some of the comments to the FDA docket on this topic imply that the ECT procedure is now a settled matter, and that low risk has somehow been proven simply by years of use. However, even many of the proponents of ECT widely admit that the electroshock device remains one of the most controversial in all of psychiatry, if not all of medicine.
Twenty-five years ago, the US government attempted to reach some kind of consensus about the controversial ECT procedure in June, 1985, through a National Institutes of Health Consensus Statement 5(11):1-23.
The NIH Consensus Statement flatly and emphatically states:
“Electroconvulsive therapy is the most controversial treatment in psychiatry. The nature of the treatment itself, its history of abuse, unfavorable media presentations, compelling testimony of former patients, special attention by the legal system, uneven distribution of ECT use among practitioners and facilities, and uneven access by patients all contribute to the controversial context in which the consensus panel has approached its task.”
Certainly, with such a controversial procedure we ought to hold the device to the highest standards. The NIH main point calls for a spotlight, not a rubber stamp.
In fact, the NIH consensus document clearly calls for the kind of regulation the FDA has been mandated to complete over these many decades, when they wrote, 25 years ago: “To prevent misapplication and abuse, it is essential that appropriate mechanisms be established to ensure proper standards and monitoring of ECT.” Clearly, these mechanisms have not been established during this past 25 years. Little has changed.
3) HIGH RISK OF MEMORY PROBLEMS ESTABLISHED BEYOND ANY DOUBT
If the FDA reclassified the ECT device, it would be considered “low risk.” However, this is its face clearly not the case.
Again, the NIH Consensus Statement established that there is a high risk of persistent memory problems even three years after electroshock for the majority of recipients.
From the NIH Consensus Statement:
“Depressive disorders are characterized by cognitive deficits that may be difficult to differentiate from those due to ECT. It is, however, well established that ECT produces memory deficits. Deficits in memory function, which have been demonstrated objectively and repeatedly, persist after the termination of a normal course of ECT. Severity of the deficit is related to the number of treatments, type of electrode placement, and nature of the electric stimulus. Greater deficit occurs from bilateral than from unilateral placement. Sine wave current has been found to impair memory more than pulsed current.
“….research conducted as long as 3 years after treatment has found that many patients report that their memory was not as good as it was prior to the treatment. They report particular difficulties for events that occurred on average 6 months before ECT (retrograde amnesia) and on average 2 months after the treatment (anterograde amnesia). Because there is also a wide difference in individual perception of the memory deficit, the subjective loss can be extremely distressing to some and of little concern to others.
Note the the word “many” about memory problems. No where does NIH say ECT is “low risk.” No informed consent material we have encountered flatly says ECT is “low risk.”
4)ECT DEVICE HAS HIGH RISK OF INDUCING ADDITIONAL TRAUMA
Individuals diagnosed with psychiatric disabilities are among the most disempowered citizens in our community, according to multiple reports. For example, Dr. Benedetto Saraceno on behalf of the World Health Organization has declared an “emergency” of human rights violations in mental health, both in developed countries like the USA, as well as developing countries. Dr. Saraceno is one of the authors about this dismpowerment in debate published by “Health Promotion International Advance Access” originally published online on December 12, 2005, 2006 21(1):70-75; doi:10.1093/heapro/dai031.
Therefore, the highest standards should be applied to a procedure that may further cause trauma to this population. In fact, not applying regulations in an equal way could further traumatize this population, by sending them a message that the elected government simply does not care about their well being.
The NIH Consensus Statement stated concerns about trauma related to ECT quite clearly. They noted that there were reports of harm, as well as some who felt helped. This diversity of responses alone shows that there is a high risk. NIH especially noted that there had been insufficient study about these hazards. From the NIH Statement:
“There are other possible adverse effects from ECT. Some patients perceive ECT as a terrifying experience; some regard it as an abusive invasion of personal autonomy; some experience a sense of shame because of the social stigma they associate with ECT; and some report extreme distress from persistent memory deficits. The panel heard eloquent testimony of these attitudes from former patients who had been treated with ECT. It is clear, however, that these attitudes are not shared by all ECT patients. The panel also heard moving testimony from former patients who regarded ECT as a wholly beneficial and lifesaving experience. There are insufficient systematic studies to permit any definitive assessment of the prevalence of these various perceptions among ECT patients.”
5) LONG HISTORY OF NEGLECT TO INVESTIGATE ECT
There are a variety of ECT device manufacturers with a wide variety of devices. The manufacturers themselves claim in their material that each device is different. In fact, according to the FDA record, ECT manufacturers themselves have filed complaints to the FDA about one another’s devices. Despite all of this, there is still no oversight.
For example, how much electrical energy delivered in joules can be given by a device? What is the upper and lower range? How does this differ from other machines? Why can this vary widely? What is the manner in which the ECT is given?
In this, one of the most controversial fields, there ought to be the highest level of oversight. A history of neglect is no argument for continuing the neglect. However, because of the disempowerment of this constituency, most States do not even require basic reporting, except for a few exceptions. One of the few states with a reporting law that is working in Texas. Their statistics show a high risk of death within 14 days following ECT.
On the federal level, MindFreedom contacted employee Shawn Terrell at the Centers for Medicare & Medicaid Services (CMS) in Baltimore about obtaining basic statistics regarding payments of ECT via Medicaid/Medicare. Mr. Terrell reported to us that he was told that there is absolutely no way for the federal government to even determine how much electroshock is paid for each year.
When it comes to the device itself, this lack of investigation is well determined.
Again, from the NIH Consensus:
“ECT has been underinvestigated in the past. Among the most important immediate research tasks are: Initiation of a national survey to assemble the basic facts about the manner and extent of ECT use, as well as studies of patient attitudes and responses to ECT. Better understanding of negative, positive, and indifferent responses should result in improved treatment practices. Identification of the biological mechanisms underlying the therapeutic effects of ECT and the memory deficits resulting from the treatment. Better delineation of the long-term effects of ECT on the course of affective illnesses and cognitive functions, including clarification of the duration of ECT’s therapeutic effectiveness. Precise determination of the mode of electrode placement (unilateral versus bilateral) and the stimulus parameters (form and intensity) that maximize efficacy and minimize cognitive impairment. Identification of patient subgroups or types for whom ECT is particularly beneficial or toxic.”
6)INDUSTRY SELF-REGULATION HAS BEEN SERIOUSLY FLAWED
In discussions with those observing the lack of regulatory oversight of the electroshock industry, one of the best analogies is the lack of regulatory oversight of the banking industry.
For instance, in several comments to the FDA, the ECT Task Force report published by the American Psychiatric Association is cited. There have been several editions.
Specifically: “Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging (A Task Force Report of the American Psychiatric Association),” by Richard D. Weiner (Author), American Psychiatric Association Committee on Electroconvulsive therapy, 2nd edition, published January 2001.
Please note that when our MindFreedom offices have contacted the American Psychiatric Association about this handbook in past years, we have been referred by the APA to a disclaimer in the beginning of the book, that this book does not officially speak for the APA’s board and officers. While we do not know if this disclaimer still applies, the point is that when confronted the APA says this book does not officially represent them.
One of the original Task Force reports actually had a model informed consent sheet claiming that only one in 200 people experienced serious persistent memory problems. A USA Today reporter determined that this number was determined anecdotally by participant Max Fink. Subsequent editions quietly dropped that reassuring statistic. But in the meantime, MindFreedom is aware of a number of informed consent sheets that utilized that number, citing the American Psychiatric Association. To repeat, the APA itself distanced itself from the book.
Again, this kind of self-regulation is reminiscent of the kind of self-regulation applied by the banking industry.
The journalist investigation of electroshock have determined many instances of this kind of self-interest. For instance, Dr. Fink had a financial connection to shock manufacturers, but this fact was not disclosed in handbooks and other material.
MindFreedom is aware of a video prepared by Dr. Fink that was used during informed consent. We’ve viewed the video and transcript. In the video, Dr. Fink goes so far as to say there is no chance of persistent loss of pre-hospitalization memories after electroshock (contracted by his own written material).
In other words, it’s no secret that the ECT manufacturers have not adequately policed and investigated their own machines. In fact, as stated, in the public record are complaints between the manufacturers themselves about each other, to the FDA.
An example of this flawed self-regulation would be in the UK with the Royal Society of Psychiatrist. Consider this study published in the British Medical Journal (BMJ 2003;326:1363 (21 June), doi:10.1136/bmj.326.7403.1363).
The title of the BMJ study is, “Patients’ perspectives on electroconvulsive therapy: systematic review” by Diana Rose et al. Their objective was, “To ascertain patients’ views on the benefits of and possible memory loss from electroconvulsive therapy.”
The report did data extraction from “26 studies carried out by clinicians and nine reports of work undertaken by patients or with the collaboration of patients were identified; 16 studies investigated the perceived benefit of electroconvulsive therapy and seven met criteria for investigating memory loss.”
Their data synthesis: “The studies showed heterogeneity. The methods used were associated with levels of perceived benefit. At least one third of patients reported persistent memory loss.”
The researchers conclusion: “The current statement for patients from the Royal College of Psychiatrists that over 80% of patients are satisfied with electroconvulsive therapy and that memory loss is not clinically important is unfounded.”
In sum, one third reporting persistent memory problems is a “high risk.” The American Psychiatric Association, in their statements to the FDA, are minimizing this high risk, just as the Royal College of Psychiatrists has done. Clearly, self-regulation doesn’t work for bankers or psychiatrists.
MindFreedom International has seen this lack of responsibility and self-regulation repeatedly among the psychiatric industry. Recently, US Senator Grassley investigated the American Psychiatric Association, and found that a great deal of their funding came from the mental health industry itself, and that this had created undue influence with the professional association.
7) THE DECISION BEFORE THE FDA IS SIMPLY WHETHER TO INVESTIGATE
In the FDA record on this topic, a number of defenders of ECT imply that the decision before the FDA is not whether or not to investigate, but the future of ECT in itself.
For example, in the American Psychiatric Association official newspaper, Psychiatric News, January 1, 2010, Volume 45 Number 1 Page 1, their article on the FDA proposed reclassification is revealing. The writer, Jun Yan, working for the APA, stated, “Small manufacturers that currently produce these devices may not be able to afford the cost of the development and submission process and cease to sell ECT devices altogether, Jennifer Tassler, J.D., APA’s deputy director of regulatory affairs in the Department of Government Relations, told Psychiatric News.”
First, it is outrageous that the APA, representing one of the most profitable industries in the USA, would argue poverty as one of the main reasons to prevent mandated regulatory oversight. The actual fact is, people diagnosed with psychiatric disabilities are, according to many studies, under-employed and frequently low-income. If anything, the systemic poverty of those with psychiatric disabilities is an argument for the FDA taking strong action, because mental health consumers tend not to have well-funded advocacy groups. Arguing that one of the ECT manufacturers might not be able to afford required investigations of the machine is a bizarre argument.
However, more importantly, statements such as the APA’s are false. We are arguing that the FDA conduct investigations of electroshock. Defenders are implying that the FDA decision to investigate electroshock in and of itself would somehow mean banning ECT. Their argument rests on the belief that ECT manufacturers would not be able to withstand the regualtory process. If that’s the case, then they ought to go to Congress to propose changing the law. But the law is the law, and it requires regulation. If they are unable to pass regulation, then they should withdraw the device from the market.
However, it is a false statement by shock defenders to claim that requiring Pre Market Approval automatically would mean ending availability of this procedure. The exception, of course, would be if the APA and other proponents are well aware that it would be impossible for the ECT device to pass current legal regulatory requirements.
To consider how wild the allegations can be come, consider that psychiatric leader E. Fuller Torrey, on behalf of the enormous Stanley Foundation, said in his comment to the FDA on this matter that critics of electroshock were led by Scientologists and other “anti-psychiatrists.” MindFreedom has zero connection to or funding from Scientology or CCHR. Of course, there are countless critics of electroshock that also have no such connection. This is not to criticize any group, but to state the truth. But in the FDA’s own record, we find this bizarre and uncivil accusation from Dr. Torrey. Our attorney, David Atkin, has a letter available on our MindFreedom web site stating we are independent.
In addition, MindFreedom is not “anti-psychiatry” and involves a number of mental health professionals, including psychiatrists. See the MindFreedom Scientific Advisory Board here: https://mindfreedom.org/campaign/development/sab
With these kinds of outlandish and false ad hominem statements from leading proponents in the FDA own’s record, how can they have the responsibility of regulating this, one of the most controversial devices in all of medicine?
On a more subtle, but still important matter, consider that a number of the comments defending the ECT device to the FDA so far imply that they officially represent organizations. For example, consider the comment from Katherine Weiss, which states that her organization is the “Veterans Administration – Boston.” The VA of course has not taken a position this matter. Dr. Weiss and others should not be implying that the VA or other taxpayer funded entities are supporting the deregulation of electroshock. Such comments should not be taken as representing their organizations, unless there is a specific and explicit statement to that effect. We have found very few such specific statements, except from the American Psychiatric Association.
We note that many of the proponents of ECT who wrote to the FDA are affiliated with institutions that in some way profit from the medical industry. There are very few comments from those who have received ECT calling for less investigation! Of course not! In fact, ECT proponents who are most likely to request ECT in the future would certainly want the devices to be investigated! Both ECT critics and recipients of ECT are united in demanding oversight.
8) GENERAL AND SPECIAL CONTROLS WOULD NOT BE ENOUGH
Proponents of electroshock may argue that with “general” or “special” controls of the ECT device, FDA reclassification could go through without any investigation. However, self-regulation by the shock industry has not worked, and the device is – as the NIH Consensus Statement said – seriously underinvestigated. Therefore it would be impossible to determine what conceivable general or special controls would be appropriate, sufficient, and scientific, without the overall investigation in question.
The ECT device must remain Class III because of a lack of investigation. This same significant underinvestigation means that there is not adequate information to even begin to create general or special controls.
If general or special controls are contemplated, there is no adequate investigation available. Just one example would be the variety of “joules” delivered by each machine. With no knowledge available, the FDA could not devise general or special controls for each manufacturer, or for each machine.
9)MULTIPLE SUB-ISSUES PROVE NEED FOR OVERALL INVESTIGATION
There are multiple serious “high risks” for the ECT device in a variety of sub-categories. Each one of these cannot be investigated in isolation. Overall investigation of the device must be completed for any of these important topics to be addressed coherently.
For example:
• ECT is sometimes still used on the very young, with developing brains. This practice is not sufficiently investigated.
• ECT among pregnant women has been shown to at times have a risk of causing damage to the fetus. (See Jacquelyn Blackstone, Michael G. Pinette, Camille Santarpio, Joseph R. Wax, “Electroconvulsive Therapy in Pregnancy” in “Obstetrics & Gynecology,” August 2007 – Volume 110 – Issue 2, Part 2 – pp 465-466.
• ECT is sometimes used involuntarily, over and against the expressed wishes of the subject. MindFreedom stands with the World Health Organization in calling for a ban of forced electroshock, but it continues, including here in the USA. For an example of this practice, in the USA, see the MindFreedom campaign about Ray Sandford, via this gateway: https://mindfreedom.org/ray For a device that can sometimes be given against the wishes of the subject, and that can cause permanent memory and cognitive problems, certainly the very highest regulatory standards should be applied. For example, how can a guardianship proceeding possibly consider whether to give involuntary electroshock, if the device itself is totally unregulated?
• ECT proponents frequently claim ECT is life-saving. However, they have never produced even one study showing in a scientific way that the risk of suicide is lowered after electroshock. Anecdotally, we are familiar with individuals who committed suicide after electroshock, attributing their disgress to the electroshock itself. For example, consider the suicide of American author Ernest Hemingway, who received forced shock and shortly thereafter told a friend he had lost his “capital,” his memory, needed for writing. He committed suicide shortly thereafter. In other words, the sub-category of suicide and ECT is underinvestigated. While there is no proof that it lowers suicide, there are stories of ECT’s trauma leading to suicide risk.
• According to reports from California about ECT administration, a large proportion of those receiving ECT are women, and a majority have been over the age of 65. Most are diagnosed depressed. There are special issues involving the disempowerment of elder women who are diagnosed with depression. This population is especially vulnerable. Of course, if older women are exploited by, for example, sales people, then the attorney general of their state can at least get involved with consumer fraud. However, when it comes to the ECT manufacturers, there is no such oversight, even over something that can potentially seriously harm their memories.
• ECT proponents frequently argue that “new improved” electroshock minimizes risks. However, certain “improvements” over the decades, such as anesthesia, means that the actual electrical energy from the device itself, measured in joules, must be higher. Some shock proponents also argue that ‘uniliateral’ shock means less memory problems. However, the unliateral shock is often done to the non-verbal side of the brain, and memory tests thend to be verbal; but if nonverbal memory tests have been used, then this improvement often does not stand.
The point here is not to absolutely settle these complicated sub-issues, but to call for an overall investigation of electroshock, that includes such proven sub-issues that remain underinvestigated. Each of these sub-issues raises a reasonable concern about high risk of the ECT device. Clearly, laws creating the FDA were meant for devices like this. Without directly investigating the overall shock devices themselves, such other important sub-issues will never be adequately investigated, either.
10)LACK OF VOICE AND CHOICE IN MENTAL HEALTH CARE
In conclusion, the larger issue that impacts ECT device regulation, is that, as shown earlier, those diagnosed with psychiatric disabilities are among the most disempowered. This means their voices are often not heard. We ask the FDA to hear these voices now. All – 100 percent – of organizations representing the voices of mental health consumers, psychiatric survivors, and mental health clients – as far as we know, are calling for more, not less investigation of the ECT device. This historic dismpowerment has been inherent and systemic in mental health care for centuries (e.g., see the book Masters of Bedlam by Andrew T. Scull et al. [1998, Princeton University Press]).
Here where the MindFreedom International office is based, in the City of Eugene, there has been widespread concern about this exclusion and disempowerment. As a result, the City of Eugene City Council unanimously passed Resolution 4989 about human rights in mental health, see: http://bit.ly/eugene-4989-1 This Resolution focused on the lack of choices that are made available to people who are experiencing severe and overwhelming despair and other mental and emotional problems in our society.
Too often, the psychiatric system has been organized based on an outdated and narrow “medical model” approach, that often ignores psychosocial, trauma and other issues. This lack of alternatives can be seen in the ECT issue. The FDA is receiving arguments claiming that ECT is a “last resort.” However, MindFreedom has seen that typically a few prescriptions are offered, perhaps some talk therapy or group therapy, and that is it. The mental health scientific literature has many more evidence-based alternatives than just drugs, talk and ECT. By investigating the ECT device, the FDA can help compare this risks and benefits of this procedure to the wide variety of proven alternatives.
On behalf of our many members who have been harmed by the electroshock device to this day, and on behalf of all people who care deeply about the human rights of mental health consumers and psychiatric survivors, I ask you to send a message of inclusion: Investigate the ECT device. Refuse to reclassify. Require Pre-Market Approval Applications. While I have not personally had ECT, I am a survivor of human rights violations in the mental health system. This is my 34th year working as a community organizer in the field of mental health. Can we finally fully include those who have received mental health care as equals? Yes we can!
Sincerely,
David W. Oaks, Director
MindFreedom International
Document Actions