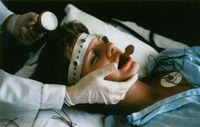
Your Deadline: Today Friday, 8 January 2010 is your last chance to demand oversight of the electroshock device in the USA. The US Food and Drug Administration (FDA) is allowing public comment about their controversial proposal to approve devices used for electroshock — also known as electroconvulsive therapy or ECT — without any testing for safety and effectiveness. Ever. It takes just a moment to comment via the web. Quantity counts!
The FDA says it may de-regulate electroshock… when it’s never regulated the device in the first place.
MFI NEWS RELEASE
Today Friday, January 8, 2010 is cut off for your comments to FDA on ECT:
It’s easier than ever to comment on the web to FDA about STOPPING their re-classification of electroshock!
You can go directly to the web form here:
At the least say:
“I oppose the FDA’s proposed reclassification of the ECT device to Class II. The FDA should investigate the ECT device for safety. The FDA should call for Pre-Market Approval Applications for the device.”
The deadline for web comments is today at 11:59 pm. Or postal mailed comments must be postdated for today (organizations need to postal mail two copies).
The FDA says numbers do count.
Of course, if you can also add specific points about why electroshock devices ought to be investigated for safety and effectiveness please do so.
But the important thing is that your comment be registered now, before the deadline!
Background:
MindFreedom International, an independent nonprofit coalition for human rights in the mental health system, launched a campaign to encourage widespread public input to stop the US Food & Drug Administration (FDA) from giving a rubber stamp to the device used for electroconvulsive therapy (ECT or electroshock).
Said David Oaks, Director of MindFreedom, “Electroshock devices are way less regulated than, say, the US banking system. Electroshock manufacturers are like the Bernie Madoff of mental health, operating unethically for decades without hardly any oversight. Now the FDA wants to rubber stamp the device and de-regulate electroshock even more. Everyone who is concerned should speak out, now!”
In Brief: USA Electroshock Regulation
Since the 1970’s, when federal law granted the FDA the authority to regulate medical devices in the United States, the machines used in electroconvulsive therapy (ECT) have been classified as high-risk, or ‘Class III’ devices. To protect the American public such devices are required by law to undergo a rigorous Pre-Market Approval (PMA) process in order to establish their safety and effectiveness.
This never happened with ECT devices. Instead, these machines were grandfathered in — having been in use for decades — and have never undergone the testing required of other Class III devices.
In April 2009, the FDA issued a notice indicating that it would finally review the safety and effectiveness of ECT devices for the first time ever.
Jim Gottstein of PsychRights explains the industry’s response: “The deadline for submissions has passed, but the manufacturers have not conducted any clinical trials, claiming they cannot afford them. They simply point to the opinions of shock doctors (including those who have financial interests in companies making electroshock machines) as evidence that shock is safe.”
Under pressure from the industry, the FDA appears likely to respond by downgrading ECT machines to Class II or “low risk.” If that happens, these dangerous machines might never be evaluated.
There is still time to take action!
Maybe because of outrage expressed by mental health advocates, including MindFreedom, the FDA opened up a “docket” for public comment until January 8, 2010. This could be the last official opportunity for years for concerned citizens to let the FDA know just how important it is to hold the ECT industry accountable for its claims that these devices are safe.
Linda Andre, author of the book “Doctors of Deception: What They Don’t Want You to Know About Shock Treatment” said: “For 30 years, FDA has been on record stating that ECT is a risky procedure which can cause brain damage and permanent amnesia, and the agency could have taken action at any time to protect patients, but it did not. A generation of patients has been subjected to an untested, unsafe procedure. It is far past time for the FDA to require that ECT be investigated for safety, and that means clinical trials, not selective literature reviews.”
You may purchase Linda Andre’s new book critical of electroshock, Doctors of Deception, via MindFreedom’s MAD MARKET, click here:
To take action about stopping the FDA from reclassifying the electroshock device, click here.
For more background info about electroshock, and links to more info click here.
Document Actions