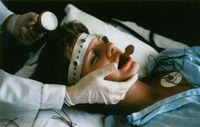
Background about the FDA proposal to de-regulate ECT device
Info Here’s more information about the Food & Drug Administration proposal to reclassify the device used for electroshock (also known as electroconvulsive therapy or ECT). A Brief History of Electroshock and the FDA: In 1976, federal law gave the FDA authority over medical devices in the United States, which had previously been unregulated. Three Classes…